Recently, on this blog, I've been musing about our foundational assumptions and how they color our thinking (for example, see here and here). In my last post, I briefly described a situation where this has affected my lab. In one of our computational studies, our model explained the experimental data better when two straightforward assumptions were made (see below). The problem was, one of those assumptions implied that Cactus can be found in the nucleus, even though it has always been conceived as a strictly cytoplasmic protein. This baseline conception colored some people's reaction so strongly as to reject our assumption, even though it was plausible.
The question I therefore raised at the end of the last post was whether our two assumptions subsequently needed to be validated experimentally? I am itching to get to that question, but before we address it, we should walk through what those two assumptions were and what experimental data are explained by the model. However, if you don't wish to read those details, here is a brief summary: The
first assumption was not only straightforward, but it also provided an obvious way to match known data. The second assumption was more
plausible than its denial, and resulted in a model that answered a
problem with our understanding of the Dorsal gradient. Now, on to the details:
First assumption: After mitosis, when the nuclear envelope reforms, the contents of the nucleus (accidentally) reflect the contents of the surrounding cytoplasm. In other words, when the nuclear envelope "encloses" the nucleus, whatever protein-sized molecules happen to be in the cytoplasm at that time can get enclosed as well.
The reason why we made this assumption is that, as soon as interphase begins, there is fluorescence in the nuclei. We saw that in live embryos expressing a Dorsal-GFP tag (see Reeves et al., 2012). There are further details to this, but suffice it to say that a simple way to solve this problem is if nuclei at the start of interphase do not begin empty.
Second assumption: If our first assumption was correct, and the nuclei do not begin interphase empty, then a chain of important implications ensues. The first implication is that both Cactus and Dorsal/Cactus complex could reside in the nucleus. That in turn implied that it is possible that some of the fluorescence we measure, either in live Dorsal-GFP embryos, or in fixed embryos fluorescently immunostained against Dorsal, originated from Dorsal/Cactus complex. And this implied that our fluorescent measurements were not of the active Dorsal gradient, but of the total Dorsal gradient (free Dorsal + Dorsal/Cactus complex). At this point, the only way to infer the active Dorsal gradient is to use our computational model.
The reason why we made this assumption was that it seemed more obvious than its denial. However, this second assumption resulted in a large boon for our model: it now was able to correctly predict the expression of genes that depend on Dorsal signaling. Without going into too much detail, we have known since 2009 that the Dorsal gradient is too narrow to express genes like sog and dpp, which have borders roughly 50% of the way around the embryo (see here for more explanation). However, our model predicted the active Dorsal gradient (as opposed to the measured, total Dorsal gradient) may indeed be broad enough to place the sog and dpp gene expression borders.
Now that we have the details of our assumptions and implications in hand, in the next post I will discuss why I no longer think experimental validation of our assumptions is necessary.
Tuesday, December 12, 2017
Monday, December 4, 2017
Validating assumptions
Oftentimes, our interpretations of our experimental data are dependent on the assumptions that we bring to the table. I have previously discussed the problem of holding unexamined assumptions (see here and here), and how they can pervade our thinking. Here I want to ask the question of how we would validate our assumptions? In particular, if we have a model that is built on assumptions, what kind of data count as evidence in favor of the model and its assumptions?
This is a pertinent question for me because, in my lab, we do both modeling and experiment, and sometimes our modeling work is not trusted in the same way that experimental results would be. We had a paper a couple of years ago (O'Connell and Reeves, 2015) that made a few new assumptions about the Dorsal/Cactus system (see here for a short intro to that system), which resulted in a much better explanation of past data, but it also resulted in a slightly novel way of looking at the system (see Figure). Specifically, we suggested that not just free Dorsal, but also Dorsal/Cactus complex and free Cactus could enter the nuclei.
This idea had not been proposed before (at least, not at this stage in Drosophila development), and reviewers were suspicious. We even had a reviewer of a subsequent manuscript try to reject that manuscript because of the assumptions we made in the 2015 paper!
Now, given that we have a wet lab, one way to test our assumptions would be to simply perform well designed experiments. But sometimes, that's easier said than done. In this particular case, because Cactus is difficult to image (both in fixed embryos, but also in live imaging), all of the proposed experiments would be indirect validations of our results, at best.
So the question is: do we actually need to validate these assumptions? That's not as radical of a question as it might sound at first. I am basically asking: weren't the assumptions already validated by the fact that the model provided a better explanation of previous data? This the question that we'll begin to look at next time.
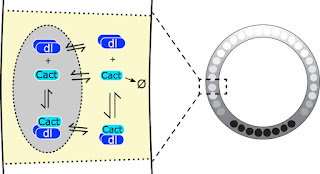 |
| In the early Drosophila embryo, Dorsal (Dl) is present at high levels on the ventral side (black nuclei on right). This gradient is established by the action of Toll signaling on the ventral and lateral parts of the embryo (not shown) causing Cactus (Cact), the inhibitor for Dorsal, to split up from Dorsal and be degraded (left). Previously, it had not been proposed that Cactus or Dorsal/Cactus complex could enter the nuclei (left). For more on Dorsal, Cactus, and Toll, see here. |
This idea had not been proposed before (at least, not at this stage in Drosophila development), and reviewers were suspicious. We even had a reviewer of a subsequent manuscript try to reject that manuscript because of the assumptions we made in the 2015 paper!
Now, given that we have a wet lab, one way to test our assumptions would be to simply perform well designed experiments. But sometimes, that's easier said than done. In this particular case, because Cactus is difficult to image (both in fixed embryos, but also in live imaging), all of the proposed experiments would be indirect validations of our results, at best.
So the question is: do we actually need to validate these assumptions? That's not as radical of a question as it might sound at first. I am basically asking: weren't the assumptions already validated by the fact that the model provided a better explanation of previous data? This the question that we'll begin to look at next time.
Monday, November 27, 2017
When diffusion works backwards
 |
| Diffusion of dye in water. |
But sometimes, diffusion seems to work backwards: it is possible for diffusion, with the help of some (chemical) reactions, to accumulate a type of molecule in one spot.
In our recent work, we found that this is precisely what is happening to the Dorsal gradient. Dorsal is a protein and transcription factor that acts as a morphogen in the early embryo. This just means that it has a concentration gradient in space and tells cells what to do in a concentration-dependent fashion. For a longer reminder of what Dorsal does, see here and here. But for now, what is important is that Dorsal is usually bound to its inhibitor Cactus, and signaling through the Toll receptor on the ventral side of the embryo causes Cactus to be degraded, so that Dorsal is free to go into the nuclei on that side of the embryo.
We have known all of this for a long time, but it wasn't until recently that we realized what this meant: that Cactus "shuttles" Dorsal to the ventral side of the embryo. This is because Dorsal/Cactus complex is lost on the ventral side of the embryo (through Toll signaling), which creates an imbalance. Because there is more Dorsal/Cactus complex on the dorsal side of the embryo, it diffuses to the ventral side. On the ventral side, the Dorsal/Cactus complexes that arrive there by diffusion also get broken up by Toll signaling (and the freed Cactus is degraded), and Dorsal goes into the nuclei. Free Dorsal protein does not diffuse back to the dorsal side because it gets stuck in the nuclei. Over time, this means more and more Dorsal gets deposited on the ventral side of the embryo.
If you've read some of my other blog posts, you'd notice that I've remarked on this before: that clearly there is more Dorsal on the ventral side of the embryo than on the dorsal side. This is why. Unfortunately, as this "shuttling" mechanism is [an ever-so-slight] shift in the standard viewpoint of what Dorsal and Cactus are doing in the early embryo, not everyone agrees this is happening. The problem is, we know the four processes in the figure above are happening, and together, those guarantee that shuttling is happening. The mechanism also explains a lot of previously confounding data.
One final note: shuttling has been discovered before in other contexts. Most notably, in BMP signaling, which is occurring in the fly embryo at the same time as Dorsal signaling, as well as in BMP signaling in vertebrates. Additionally, shuttling has been hypothesized to be occurring upstream of Toll signaling.
Wednesday, July 12, 2017
Models should match observations
Ever since Galileo dropped two objects out of the Leaning Tower of Pisa (according to apocryphal accounts), it has been recognized that our scientific theories should be tested by observation. Sure, you could just make something up about how things work, but eventually someone will come along and just look to see if you're right.
Last week I wrote about an example in modern-day science where this hasn't happened. It was a small example...we are not talking about anything as momentous as testing the acceleration of gravity. But the example illustrated a point: sometimes ideas get so stuck in our heads that it may take a while before we realize that the observations are saying something different.
Here is another example. Because of the action of the Dorsal nuclear concentration gradient (see here for more information about Dorsal), different genes are expressed in different locations around the circumference of the embryo. For example, the gene snail (sna) is expressed on the ventral-most 20% of the embryo (see figure below; by convention, the dorsal side is up and the ventral side is down). But measurements of where other genes are expressed were not so easy to do. Before we performed quantitative experiments in cross-sectioned embryos (where you could see the entire circumference of the embryo), it was thought that sog extended from about 25% to 70% around the embryo (see lavender colored arc on left figure). It was also thought that dpp was only in the cells on the dorsal-most 30% of the embryo.
However, after we began to make quantitative observations (using fluorescence) in cross-sectioned embryos, we could easily see that sog extends from 20% to 50% (see green fluorescence in middle figure and green curve in graph on right), while dpp takes up almost the entire dorsal half of the embryo (yellow fluorescence and yellow curve). However, even though this has been known since 2009, scientists are still publishing illustrations like the one on the left.
Last week I wrote about an example in modern-day science where this hasn't happened. It was a small example...we are not talking about anything as momentous as testing the acceleration of gravity. But the example illustrated a point: sometimes ideas get so stuck in our heads that it may take a while before we realize that the observations are saying something different.
Here is another example. Because of the action of the Dorsal nuclear concentration gradient (see here for more information about Dorsal), different genes are expressed in different locations around the circumference of the embryo. For example, the gene snail (sna) is expressed on the ventral-most 20% of the embryo (see figure below; by convention, the dorsal side is up and the ventral side is down). But measurements of where other genes are expressed were not so easy to do. Before we performed quantitative experiments in cross-sectioned embryos (where you could see the entire circumference of the embryo), it was thought that sog extended from about 25% to 70% around the embryo (see lavender colored arc on left figure). It was also thought that dpp was only in the cells on the dorsal-most 30% of the embryo.
 |
| Patterns of gene expression along the dorsal-ventral axis in the early Drosophila embryo (about 2.5 hours old). Illustration on the left (adapted from Stathopoulos and Levine, 2004) depicts sog as extending from 25% to 70% around the embryo (ventral-to-dorsal). dpp is shown as taking up the dorsal-most 30% of the embryo. More recently, fluorescent imaging in cross sections has become possible. The image in the middle is of a cross-sectioned embryo with several different genes detected by fluorescence. In particular, sog extends from 20% to 50% of the embryo circumference, while dpp takes up almost the entire dorsal-half of the embryo (adapted from Reeves and Stathopoulos, 2009). Both of these observations (plus those regarding other gene expression patterns) can be quantified and plotted as graphs (on right; adapted from Reeves et al., 2012). A border of a gene is defined as when it drops to 50% intensity. |
However, after we began to make quantitative observations (using fluorescence) in cross-sectioned embryos, we could easily see that sog extends from 20% to 50% (see green fluorescence in middle figure and green curve in graph on right), while dpp takes up almost the entire dorsal half of the embryo (yellow fluorescence and yellow curve). However, even though this has been known since 2009, scientists are still publishing illustrations like the one on the left.
Wednesday, July 5, 2017
Shifting paradigms
In biology, as in all sciences, sometimes an idea gets so big, and so ingrained/entrenched in the culture, the original work does not need to be cited anymore. (Unfortunately, sometimes this means we often forget where the idea originally came from.) This idea becomes so pervasive, we believe it without even thinking about it. It becomes a fall-back idea, a foundation or bedrock, so to speak. Every new discovery is measured against it. Such an idea is called a paradigm.
However, sometimes, upon further review years later, we find that the original research that started the paradigm was flawed. Or the paradigm rests on a particular interpretation of the data from the original research, and not on the data themselves. In that case, the paradigm may shift. But because paradigms are so pervasive, it might take a lot of work and a long time for the shift to occur.
Now, to be clear, I am not claiming that any of the work I have been a part of constitutes a paradigm shift. Not at all. Usually, we reserve that moniker for truly momentous changes in an entire field of science, such as quantum mechanics or relativity. But some of our observations have shown that common illustrations of fly embryos, which have been in people's minds for decades, do have some inaccuracies.
For example, consider the following illustration of a cross section of a fly embryo that is about 2.5 hrs old (left side of jpg below). The green represents the presence of the Dorsal protein (see here for more information about Dorsal). The common thought is that, as Dorsal enters the nuclei on the ventral side (bottom half of illustration), it depletes the surrounding cytoplasm of Dorsal. Hence, the cytoplasm around the ventral nuclei are dark. In contrast, Dorsal protein does not enter the nuclei on the dorsal side of the embryo (top half of illustration), so the nuclei are dark, but the surrounding cytoplasm is bright.
In theory, this "default view" of what happens with Dorsal should last only as long as it takes for someone to just look and see. In 2009, when I was a postdoc in Angela Stathopoulos's lab at Caltech, we published the first quantifiable (i.e., fluorescent) images of Dorsal-stained, cross-sectioned embryos (for example, see jpg above, right side). In several publications thereafter, we always saw the same thing: the cytoplasm is not bright on the dorsal side. In fact, there is no doubt there is just more total Dorsal protein on the ventral side. Yet, these illustrations of Dorsal in the embryo still persist today. I guess it just takes some time and effort for the "default view" to get out of our collective head.
However, sometimes, upon further review years later, we find that the original research that started the paradigm was flawed. Or the paradigm rests on a particular interpretation of the data from the original research, and not on the data themselves. In that case, the paradigm may shift. But because paradigms are so pervasive, it might take a lot of work and a long time for the shift to occur.
Now, to be clear, I am not claiming that any of the work I have been a part of constitutes a paradigm shift. Not at all. Usually, we reserve that moniker for truly momentous changes in an entire field of science, such as quantum mechanics or relativity. But some of our observations have shown that common illustrations of fly embryos, which have been in people's minds for decades, do have some inaccuracies.
For example, consider the following illustration of a cross section of a fly embryo that is about 2.5 hrs old (left side of jpg below). The green represents the presence of the Dorsal protein (see here for more information about Dorsal). The common thought is that, as Dorsal enters the nuclei on the ventral side (bottom half of illustration), it depletes the surrounding cytoplasm of Dorsal. Hence, the cytoplasm around the ventral nuclei are dark. In contrast, Dorsal protein does not enter the nuclei on the dorsal side of the embryo (top half of illustration), so the nuclei are dark, but the surrounding cytoplasm is bright.
In theory, this "default view" of what happens with Dorsal should last only as long as it takes for someone to just look and see. In 2009, when I was a postdoc in Angela Stathopoulos's lab at Caltech, we published the first quantifiable (i.e., fluorescent) images of Dorsal-stained, cross-sectioned embryos (for example, see jpg above, right side). In several publications thereafter, we always saw the same thing: the cytoplasm is not bright on the dorsal side. In fact, there is no doubt there is just more total Dorsal protein on the ventral side. Yet, these illustrations of Dorsal in the embryo still persist today. I guess it just takes some time and effort for the "default view" to get out of our collective head.
Tuesday, June 27, 2017
The Happy Path and genome sizes
In software engineering, the Happy Path refers to the case when execution of the code happens with no problems or exceptions or possible errors. This scenario could also apply to other areas of engineering. For example, in chemical manufacturing, the unit operations are designed to give the desired output when the inputs are just as expected. But sometimes, disturbances upset the unit operation, so operating parameters like steam pressure, cooling water flow, etc., must be changed "on the fly" to make sure the product is up to specification. (That is what process control is for.)
The same principle is also true of other engineered systems. For example, while a car is designed to operate in a wide range of weather conditions, most of the time, you are on the Happy Path. But when it rains, it is a good thing the engineers built a subsystem of the car (wipers) to keep the water on your windshield from obscuring your view. An even more extreme case is your airbag. Hopefully, you'll never need to see your airbag in operation, but when you get off the Happy Path by crashing into something, you'll be glad the airbag is in place. Indeed, I am sure that careful inspection of the car's components, including the programming in the car's onboard computer, will show that a sizable fraction of the car is dedicated to situations that are not on the Happy Path.
In biology, "Happy Path" could be envisioned as when cells are grown under pristine laboratory conditions. If cells are kept at the just right conditions, with the just right amounts of nutrients, they will only execute the most basic sets of code and subroutines (the Happy Path). Under these cases, researchers have been able to strip down the genome to about 10% of its normal size. On the other hand, under wild (or uncontrolled) conditions, cells might face a myriad of challenges, and must execute various subroutines to proceed with growth/division. In this way, the vast majority of DNA code within the cell is there to ensure the cell continues to grow and divide, even when faced with unpredictable, suboptimal conditions.
Both the cell and man-made systems display this hallmark of complex, engineered systems: that a large fraction of the system's make-up is in place to provide robustness in the face of many disturbances.
The same principle is also true of other engineered systems. For example, while a car is designed to operate in a wide range of weather conditions, most of the time, you are on the Happy Path. But when it rains, it is a good thing the engineers built a subsystem of the car (wipers) to keep the water on your windshield from obscuring your view. An even more extreme case is your airbag. Hopefully, you'll never need to see your airbag in operation, but when you get off the Happy Path by crashing into something, you'll be glad the airbag is in place. Indeed, I am sure that careful inspection of the car's components, including the programming in the car's onboard computer, will show that a sizable fraction of the car is dedicated to situations that are not on the Happy Path.
In biology, "Happy Path" could be envisioned as when cells are grown under pristine laboratory conditions. If cells are kept at the just right conditions, with the just right amounts of nutrients, they will only execute the most basic sets of code and subroutines (the Happy Path). Under these cases, researchers have been able to strip down the genome to about 10% of its normal size. On the other hand, under wild (or uncontrolled) conditions, cells might face a myriad of challenges, and must execute various subroutines to proceed with growth/division. In this way, the vast majority of DNA code within the cell is there to ensure the cell continues to grow and divide, even when faced with unpredictable, suboptimal conditions.
Both the cell and man-made systems display this hallmark of complex, engineered systems: that a large fraction of the system's make-up is in place to provide robustness in the face of many disturbances.
Subscribe to:
Posts (Atom)


